Playing chicken with our health
Highlights
- The FDA, the CDC, and pharmaceutical manufacturers knew that vaccinating for chickenpox would lead to more shingles later in life, but they did not disclose this information. Instead, they simply created a new vaccine for shingles.
- The justification for plowing ahead with the chickenpox vaccine was not about health, it was about money. CDC used statistical projections that the shot would help the economy to support their recommendation at a time many parents and doctors thought the shot was unnecessary at best, or harmful at worst.
- The chickenpox vaccine was developed using fetal cell lines (one with a known mutation), and testing included children who were institutionalized for “mental retardation,” as well as children with cancer.
“If you could prevent shingles, why wouldn’t you?”
These are the bold words on the shingles vaccine homepage. You might wonder why an article shining a light on the chickenpox vaccine is starting out quoting a shingles marketing campaign. We won’t leave you in suspense: It’s because rolling out the chickenpox vaccine caused more shingles cases. And, as you’ll learn, the federal government, the drug manufacturer Merck, and the scientific community all knew this would happen before the chickenpox vaccine was licensed and recommended.
That’s a gutsy statement of fact, and we’re ready to back it up. Read on.
What is chickenpox?
Before we dive into the policy, you need a basic background of what we’re dealing with. In short, chickenpox, which is known by the medical name varicella, is an itchy rash with a slight fever that typically occurs in young children. It’s transmitted via respiratory droplets or through direct contact with the blistery rash. People may also experience symptoms like tiredness and loss of appetite. Most children recover without issue within two to four days.

Complications are rare in healthy children, but are more frequent in people who are older than 15, younger than 1, or immunocompromised.1 Prior to 1995 when the vaccine was licensed in the U.S., there were about 100 deaths a year attributed to varicella, with approximately four million cases of chickenpox annually. While CDC data from 1990-1994 indicates that adults older than 20 years of age made up only 5% of varicella cases, greater than half of all varicella deaths occur in that age group.2 Fatality is very rare in children, and 15-25 times more likely in early adulthood than in childhood. 3
How did chickenpox get its name?
Just like influenza and rubella, the illness we call chickenpox has gone by many names and been confused with other illnesses scientists now see as distinct. “Modern Home Medical Advisor” tells us that “all sorts of names have been applied to chickenpox, not only by the public but also by physicians. In some parts of the country, it is known as water pock, glass pock, sheep pock, and crystal pock. These names are related either to some resemblance of the blisters, to similar conditions occurring in animals, or, in fact, to the resemblance of the blisters to water or crystal. In 1730, British physician Thomas Fuller noted that the pustules looked as if a “Child had been picked by the Bills of chickens.” 4
Also, “in the dictionary of the English lexicographer, Samuel Johnson (1709-1784), it is mentioned that chickenpox is so called “from its being of no great danger.”5 Scientifically, the condition is called varicella, but it was also called variola spuria, or spurious smallpox because it was so frequently mistaken for smallpox.”i As two medical researchers have put it, “These infectious diseases not only share a common “pox” nomenclature, but are also closely interconnected in medical history.”
While chickenpox, smallpox, cowpox, monkeypox, and the “great pox” (syphilis) may share the pox name, they don’t all share origins. “Monkeypox, smallpox, and cowpox are caused by orthopoxviruses, whereas syphilis is a bacterial disease (Treponema pallidum) and chickenpox is caused by varicella zoster virus (VZV).”6 The “pox” they have in common is the visual sign of illness, which has historically meant a blister, or “eruptive sore” on the skin (but the term is also used as a curse).
There was no clear scientific distinction between chickenpox and smallpox until the late 1800s, but the clinical delineation was made more than a hundred years prior in 1767 when English physician William Heberden noted in his paper “On the Chicken-pox” that having had chickenpox did not prevent one from contracting smallpox. 7 Prior to Heberden’s observation, chickenpox was simply characterized as a milder form of smallpox. 8
Three events in the late 1800s gave scientific confirmation that chickenpox was indeed not smallpox. “In 1875, [Rudolf] Steiner inoculated healthy volunteers with vesicular fluid from individuals with active varicella infection, thus demonstrating that chickenpox is caused by an infectious agent. In 1892, a case presentation by Osler emphasized that smallpox and chickenpox were not the same disease, and that infection with one did not provide immunity to the other, solidifying Heberden’s initial assessment that chickenpox and smallpox were unrelated. A few years later, Ernest Tyzzer developed a bedside test to distinguish smallpox from chickenpox based on the presence of multinucleated cells in varicella lesions that were not found in smallpox.” 9
Despite being scientifically indistinguishable from smallpox until the late 1800s10, chickenpox was described in the 1942 edition of “Modern Medical Home Advisor” as “not a particularly serious disease, since it is seldom associated with high fever or with much depression. … With the exception of German measles, chickenpox is probably the mildest of all the infectious diseases that attack children.”xi Today, although it is no longer mistaken for a milder form of smallpox, the general consensus is that chickenpox, while highly contagious, is still a mild childhood illness. 11

As an example of just how mild this illness is, Scholastic published a children’s early reader book called “Itchy, Itchy Chicken Pox” in 1992 for early readers. One educator who did a read-aloud on YouTube called it a “funny, simple” book and asked if kids could relate to that “itchy feeling you get when you have the chickenpox.” She posted in April 2020, at the height of the declared COVID pandemic, and generalized germ fear didn’t stop her from posting.12

Of note, according to the January 29, 1999, edition of the CDC’s “Morbidity and Mortality Weekly Report” (MMWR), chickenpox became a nationally notifiable illness in 1972 (coincidentally when the first viable vaccine was proposed for study in the U.S.), but was deleted from the weekly CDC morbidity report in 1981. In 1982, states were once again encouraged to report cases annually to the CDC’s National Notifiable Disease Surveillance System (NNDSS). Originally, 46 states and the District of Columbia submitted data, but by 1997 only 20 states were reporting data to the CDC. Deaths from varicella were not reportable until 1999 (after the vaccine was released).13 The lack of required reporting before a vaccine existed again reveals the mild nature of the illness.
So what is shingles?
Shingles, known as herpes zoster in the medical world, is attributed to the same virus that causes chickenpox. It is believed after chickenpox infection, the virus becomes “latent” and nestles in next to your nerves. Today, it’s fairly common knowledge that chickenpox, like other herpes viruses, causes a latent infection as the virus settles in the sensory nerve ganglia and can later reactivate resulting in herpes zoster (HZ), also known as shingles.14 Both the medical name (herpes zoster) and the common name (shingles) is derived from the idea of a kind of rash that spreads around the midsection like a belt. “Herpes” is derived from a Greek word meaning “to creep,” and “zoster” is derived from Greek and Latin words for belt. Likewise, “shingles” comes from another Latin word for “girdle.”xiii
The scientific discovery that the varicella zoster virus leads to both chickenpox infection and shingles has been described as “one of the most exciting adventures in medical virology.”15 The breakthrough was made in 1892 when James Von Bókay “noted the occurrence of both disorders at the same time in homes of his patients. He recognized the similar appearance of the skin findings and hypothesized that the same virus produced the two, thus setting the stage for experimental analysis.” 16
In 1965, Edgar Hope-Simpson noted that zoster (shingles) never occurred in epidemics and guessed “that zoster was due to reactivation of latent virus and that viral latency was maintained by repeated exposure to exogenous (external) virus.”17 In other words, the sleeping shingles virus was awakened when older people were exposed to chickenpox out in the world.
Historically, shingles occurs in adults and even though the illness is associated with the same VZV virus as kids’ chickenpox, the result in adults is not mild. The resulting shingles rash is known to be quite painful and lasts for 7-10 days, with healing taking as long as 2-4 weeks.18 Besides the itching, burning, stabbing, shooting pain of the rash, other symptoms include fever, chills, headache, tiredness, sensitivity to light, and stomach upset.19
No scientific consensus exists yet on what controls latency (inactivity), but it’s believed that factors include a compromised immune system, age, illness, trauma, and stress. Approximately 10% of the people who contract chickenpox will later develop shingles, usually after the age of 50. Unfortunately, individuals can experience shingles more than once.20
Interestingly, an individual with shingles is contagious, but contact with the rash can only result in chickenpox, not shingles. Approximately 10%of shingles cases will experience a debilitating side effect known as postherpetic neuralgia (PHN), where the nerve pain persists after the rash has disappeared. PHN can last for weeks or months, sometimes a year or longer.
Who wants chickenpox?
Prior to the introduction of the vaccine in 1995, there were approximately four million cases annually in the United States, a number estimated to be equivalent to the annual birth cohort. In other words, everyone got chickenpox at some point in their lives, usually early.
As with other childhood illnesses typically referred to as “mild” in the pre-vaccine era, (like measles) children acquire lifelong immunity to natural chickenpox infection. It’s significant to note that, “as with other viral diseases, re-exposure to natural (wild) varicella may lead to reinfection that boosts antibody titers without causing clinical illness or detectable viremia (infection).”21 Nature gives us natural boosters to chickenpox infection and, as you read above, stops shingles from emerging.
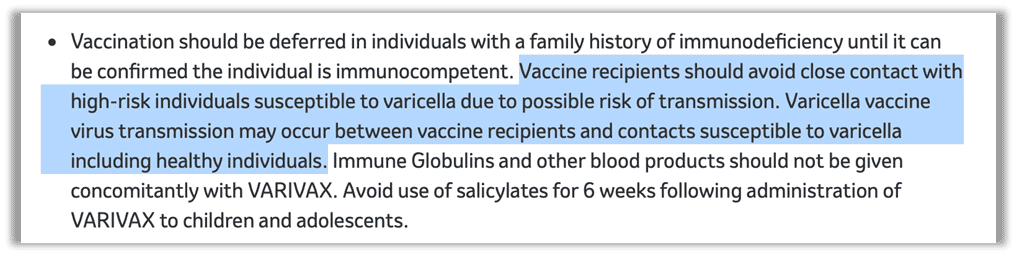
As is the case with measles, natural infection with chickenpox offers benefits that cannot be found with a vaccine and, more importantly, the absence of chickenpox has created an epidemiological shift — a change in the patterns of disease occurrence in a population over time – with significant negative consequences. Dr. Thomas Cowan asserts, “There is evidence that our bodies need exposure to certain childhood illnesses in order to establish the foundation of lifelong health… a largely benign illness like chickenpox reduces the risk of brain cancer, while the vaccine increases the risk of shingles.”22 23 Further, one of the oft-overlooked benefits of chickenpox is the visual sign of illness — the rash — a signal to isolate and stay away from those susceptible to a more severe infection if exposed. Merck’s application for licensure to the federal government revealed that varicella vaccinees are able to transmit the vaccine strain of the virus to others, including the immunocompromised, despite the absence of a rash. Today, Merck still warns about the possibility of transmission of the vaccine strain to close contacts on their website and in the vaccine insert., This should give employers and schools pause before requiring or mandating varicella vaccination. Our children won’t have those benefits if we keep vaccinating with the chickenpox shot.
“American children are now so chronically ill that we must come to new understandings about medicine, health, and disease. We are locked in a cage of symptom suppression like someone trying to stem a flood by holding a finger in the dike. Acute childhood illnesses like measles and chickenpox teach a child’s immune system how to respond. Perhaps more importantly, they teach a child how to engage with her body in a powerful and intense way — a process through which a child becomes herself and learns to make her body her own. Preventing or trying to completely control this process will result in a lifelong battle against the self — that is, an autoimmune disease.” – Dr. Thomas Cowan, Vaccines, Autoimmunity, and the Changing Nature of Childhood Illness.
Cowan continues, “While nobody loves getting sick, or seeing their children sick, nearly everyone experienced full recovery, and gained lifelong immunity. Many people actually harbor warm memories of being intently cared for — or caring for their own children — and the particular bonding that occurs when one nurses another who is acutely ill.”xviii He goes on to further explain the bonding that happens between parent and child while we guide them through illness, describing it as “an experience that builds their trust in us as parents and deepens our understanding of our children and their needs. When we always strike preemptively by overvaccinating, overpathologizing, and overtreating children from a very young age, we forfeit the opportunity to develop a deep understanding of the people we love and want to know, best. If nursed through an illness, once the child is well, he’s felt his parent’s presence when it was needed. And the parent has developed experience on which to draw when accompanying him through other ‘battles’ — whether behavioral, emotional, or medical. Wise parents know that it’s not our job to orchestrate the world so that our children never encounter any conflict or challenge; it’s our job to be present with them, aware, and available, as they make their way through a given experience from which they have the opportunity to grow.”
Chickenpox parties were more common in older generations, but still happen today, as a quick internet search will reveal. The “pox parties” made sure chickenpox happened during childhood. (Most parents understand that contracting it as an adult is not as tolerable.) And the parties allowed parents to coordinate exposure for the sake of convenience, whether for time or health reasons. And it’s not just “crunchy” parents pushing pox parties. “In 1935, University of Pennsylvania professor Theodore Ingalls, whose wife had lost a child after contracting rubella during pregnancy, tried to get the city of Philadelphia to organize chickenpox and rubella parties so children could be exposed in a controlled way.”24 Chickenpox parties represent a collective knowledge about illnesses passed down through generations that pharmaceutical science overlooks and shuns.
Who wants a chickenpox vaccine?
Cowan says in his book, he was “shocked” when the chickenpox vaccine was licensed. “I couldn’t imagine the rationale for a vaccine that doesn’t even offer lifelong immunity for a basically insignificant illness.” He wasn’t alone. The one-two punch of adding a questionable hepatitis B vaccine and then varicella to the recommended childhood schedule was an eye opener for many parents and physicians.25
Again, the questions weren’t limited to worried parents at playdates. There was serious, published, scientific debate about whether a chickenpox vaccine was a bad idea because it was understood shingles cases would go up. The questions were so strong that it delayed licensure of the vaccine for over a decade.
To cut to the chase, the justification for plowing ahead with the chickenpox vaccine was not about health, it was about money. And although pharmaceutical companies did profit, the roots of the chickenpox vaccine policy go much deeper.
For much the same reason moms and even doctors promoted chickenpox parties, women in the workforce accelerated the acceptance of a vaccine: convenience. As researcher Arthur Allen stated, “the vast majority of parents remembered it as a normal passage of childhood, [but] in 1995, the year the chickenpox vaccine was licensed, most women with children worked and could not afford to stay home for a week with a sick child even if they wanted to.” The chickenpox vaccine was born out of the women’s rights movement, just like its ancestor the rubella vaccine. It was about giving women “freedom” and “empowerment” to be in the workforce, and it was further justified by statistical fiction that the shot would save Americans money (claiming prevention was less expensive than experience). It was never about health. Women were in the workforce and they wanted to stay there. With two parents working, who would stay home with a sick child? And for those who questioned the need for a shot for a mild illness, we had a stat for that.
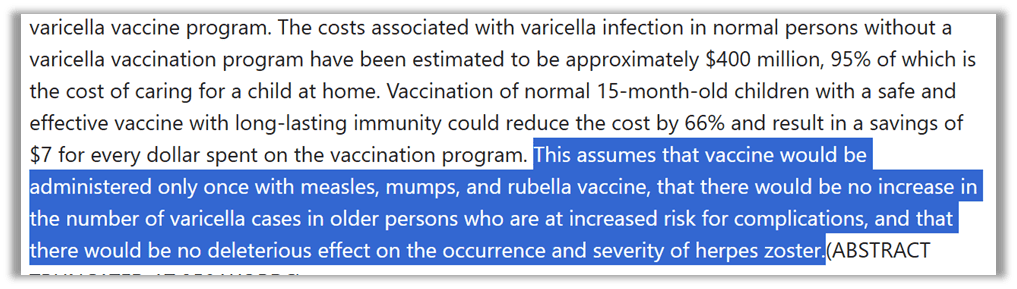
You can see the assumptions on which cost saving rested: that there would only be one shot (we now have two), and that the vaccine wouldn’t cause more complications or shingles (which it does).26
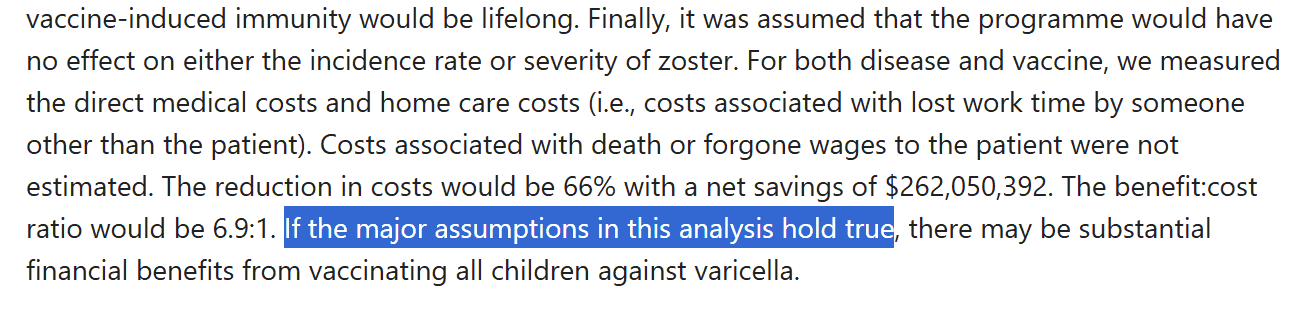
The Vaccines for Children (VFC) program sealed the deal on the chickenpox economic argument. When the federal government wants to push a policy on states that it has no jurisdiction to move, it uses money – our very own tax dollars – as the carrot on the stick. Under the federal VFC program, states mandate shots because it qualifies them for federal funds.27
After the national rollout of the polio vaccine, states learned the federal government could and would fund immunizations. “When Kennedy signed Public Law 317 he expressed much loftier goals. There was no reason, he said, that any child should die of smallpox, diphtheria, polio, pertussis, or tetanus. Initially the funding of PL 317 was supposed to be a one-off affair. But you couldn’t expect to permanently whip any of these diseases just by sweeping in to vaccinate and then leaving, so the bill became a mechanism for purchasing vaccine for local health departments. But there was still not enough money to support the infrastructure of immunization, though, such as clinic staffing.” When Bill Clinton took office, the administration embarked on a major overhaul of the program, turning Kennedy’s temporary PL 317 into a permanent mandatory spending on VFC.
In 1994, Congress enacted the VFC program, which obliged the federal government to finance vaccinations for children under 19 for all vaccines recommended by the ACIP. States weren’t required to implement the recommendations. But no state wanted to skip it and end up as a bad example if illness soared and lawmakers declined federal funds. You can imagine that would be political suicide. Whether or not it was worth vaccinating against chickenpox, at the end of the day, South Dakota would be embarrassed by chickenpox epidemics if North Dakota no longer had them. Mandating a recommended vaccine brought money into a state’s immunization program. And this is how federal “recommendations” become state law.
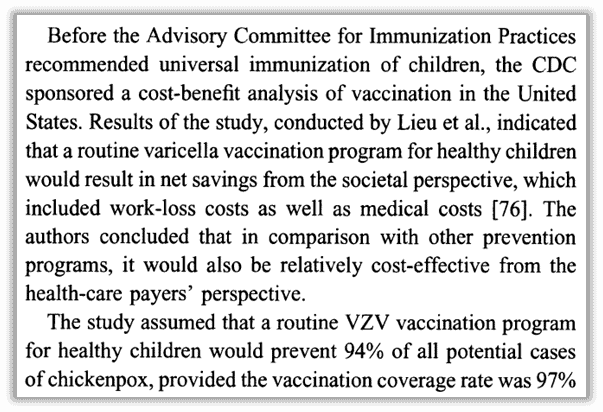
You can find claims that universal chickenpox vaccination has saved health care costs overall, but three honest scholars admitted in a 2014 peer-reviewed analysis titled “Successes and challenges in varicella vaccine,”28 that “cost-effectiveness studies evaluating universal childhood varicella vaccination programs are hard to interpret,” largely because the illness is so mild that countries still don’t track it. After thorough review they determined, “Few economic analyses conducted in the USA, Australia and Europe have shown varicella vaccination programs to be cost-effective from a health care perspective and even cost saving from a societal perspective.” Their work points out the shortcomings and challenges with published analysis.

Why did the chickenpox vaccine take 29 years to make and license?
The first attempts at a chickenpox vaccine were made by Merck in 1966.29
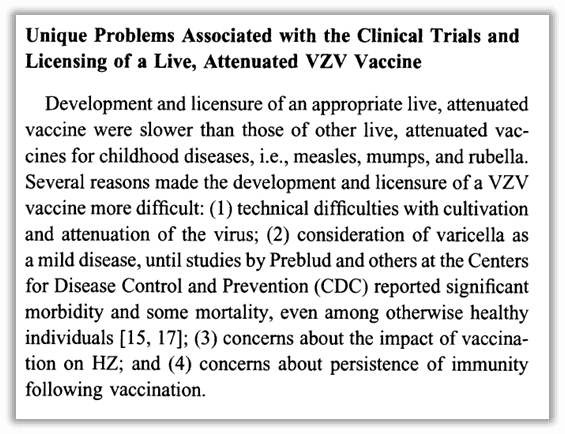
In a “state of the art clinical article” about the varicella vaccine, the “unique” problems associated with its development were summed up (to paraphrase): it was tricky to make, chickenpox was considered mild until CDC scientists convinced people otherwise, there were concerns about the vaccine’s impact on shingles cases, as well as concerns about how long a vaccine could last.30 Despite these obstacles and controversies, the author also points out that “the primary goal of the VZV vaccine development program by [Merck] in the United States was to obtain data to support universal immunization of children.”
You might wonder why scientists wanted to create a chickenpox vaccine if it was so difficult and controversial. Research began in the 1970s by a man named Michiaki Takahashi.31 A Japanese scientist working temporarily in Texas, he was inspired to create a vaccine when his 3-year-old son got chickenpox and had a rare complication.
Anne Gershon, one of the world’s experts on varicella who closely tracked the development of the vaccine closely and authored papers on it, mused that before the vaccine announcement in 1974, “there seemed to be little or no interest in producing it. Not only was varicella considered a mild disease and therefore not really worth preventing, but the ability of VZ virus to cause latent infection was well known and was considered a barrier to active immunization.”32 Takahashi’s announcement was met with “ambivalent reactions.” Similar to rubella, measles, and mumps, there was no market for it, but more importantly it was considered potentially dangerous.
The chickenpox vaccine was made by “weakening” a live virus. This is a technique called “attenuation” where an original virus sample is serially injected into cell culture after cell culture to force it to mutate so it will adapt to the controlled environment and hopefully not thrive in humans after mutation.
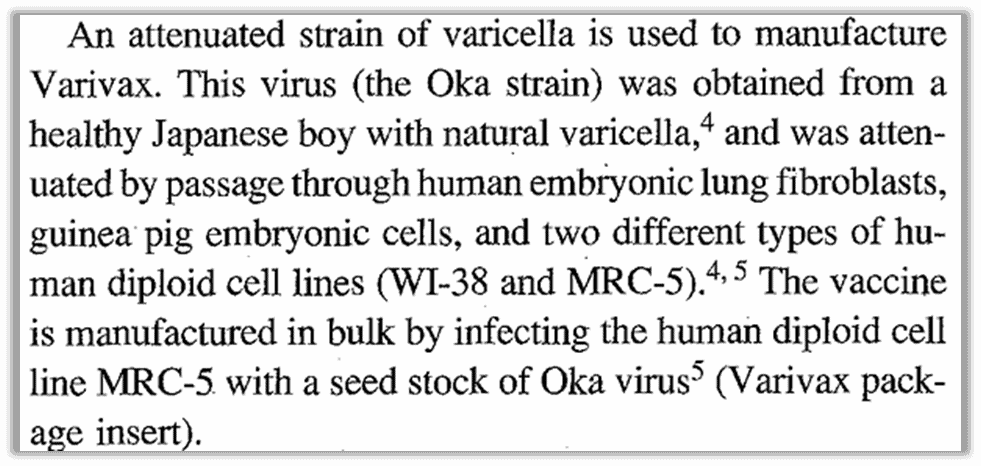
The 1995 “Summary for Basis of Approval Agreement” (SBA) between the FDA and Merck described the vaccine virus as weakened by “passing” the virus taken from a young boy through a series of three types of cells: two human, one animal. This included lung cells from a human embryo (MRC-5), then guinea pig embryo cells, then finally human cells.

What does this mean? Scientists took a sample from a young boy with chickenpox who was otherwise healthy. They got a batch of MRC-5 cells, which are lung cells taken from a 14-week old male embryo, and infected the cells with the virus sample.
The virus was hard to work with. In the U.S., Merck tested several formulations of the vaccine over 14 years. “Each time improvements were made to the process, several thousand children were tested for safety and immunogenicity.”33 (Don’t you wish a vaccine information statement would print the number of children tested in clinical trials?) Gershon wrote in 1985 while the vaccine was anticipating licensure in the United States, “There is no suitable animal model for VZ virus infection; therefore, the initial vaccine testing had to be carried out directly in humans.” Gershon explains that “this was done in a stepwise manner” in healthy children, then immunosuppressed children, then kids with cancer. Those healthy volunteers came (at least in part) from “an institute for the mentally retarded,”34 following the common and accepted custom of using our most vulnerable children for scientific experimentation, as with with rubella, hepatitis B, mumps, measles, polio, and RSV.
“Its use was a great departure from tradition for two reasons. It was the first live vaccine administered to children that was prepared from a virus that causes latent infection. It was also a live virus vaccine specifically to be given to immunocompromised patients.”35
FDA senior research investigator Philip Krause was quoted telling the Vaccines and Related Biological Products Advisory Committee (VRBPAC), “We are fairly comfortable with the clinical data. We believe they have been analyzed as carefully as they can be.”36
An article published in the Journal of the American Medical Association (JAMA) warns us, “Almost from the beginning, problems with manufacturing, efficacy, and safety have plagued this biological agent.” (emphasis added)37
Efficacy
The “Summary for Basis of Approval Agreement” states that “wild-type varicella is endemic in the U.S, so some children participating in efficacy trials were undoubtedly re-exposed to chickenpox when their friends or siblings became infected. Such inadvertent exposure could have boosted the vaccinee’s immune response, resulting in increased serum antibody titers and potentially extending the subject’s immunity to varicella. Only after most children are immunized with VARIVAX will this booster effect diminish and an unequivocal analysis of the vaccine’s long-term efficacy become possible.”
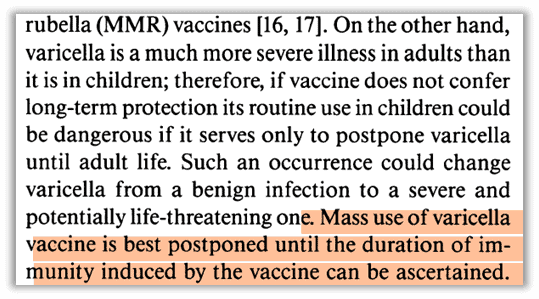
Gershon expressed caution before the licensure of the chickenpox vaccine in the U.S. based on uncertainty of duration. If the vaccine only pushed chickenpox illness into adulthood, it would be “dangerous” and “could change varicella from a benign infection to a severe and potentially life-threatening one.”
Gershon has also pointed out, “there is no useful animal model of VZV disease, and so it has been difficult to study protection that develops after vaccination in any degree of detail.”38
But even now, thirty years later, the CDC admits that duration of protection is unknown because the initial studies took place “when infection with wild-type varicella was still very common.”39

“Outbreaks of breakthrough varicella among vaccinated children in day care centers and schools were reported in several U.S. states since the introduction of the one-dose universal vaccination program…. It has been supported that vaccination at a younger age, time since vaccination, vaccination within 28 days post-MMR, and a history of asthma, eczema or steroid intake may be associated with varicella vaccine failure.”40
Shedding
In addition to shifting the disease to older ages, It is well known that the chickenpox vaccine sheds, though in medical literature you may see it described as “environmental release.”41 This makes one wonder why the vaccine shedding does not cause the same “exogenous boosting” like natural infection to ward off shingles in older populations?

Effect of the vaccine rollout on chickenpox and shingles cases
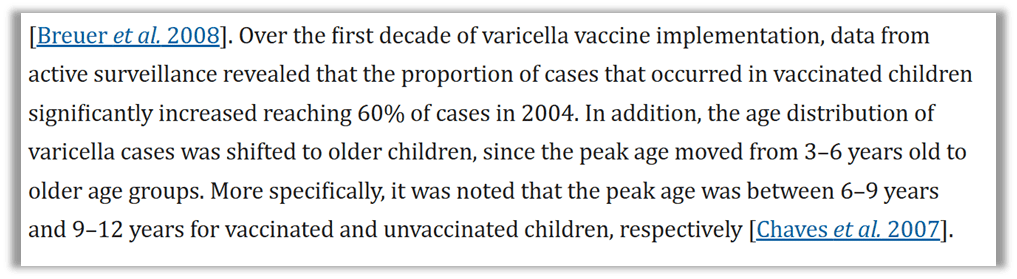
A common occurrence after the introduction of a vaccine is that the illness isn’t totally prevented, it’s simply delayed. It’s well understood that the introduction of the chickenpox vaccine suppressed the illness in our youngest children and shifted the sickness to adolescents and adults.42
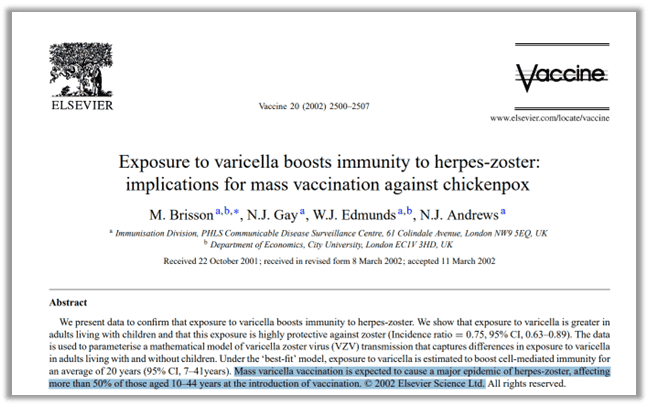
The chickenpox vaccine was introduced in the 1990s and by the 2000s we saw a “massive increase” in the number of cases of shingles, which until then had been “uncommon” in healthy adults43. A journal called “Vaccine” published a peer-reviewed study in 2002 that predicted, “Mass varicella vaccination is expected to cause a major epidemic of herpes-zoster [shingles], affecting more than 50% of those aged 10-44 years at the introduction of vaccination.”44 (emphasis added). The title of the article shouted what many already knew but didn’t want to talk about: Natural chickenpox infections circulating in a population act as a natural booster to prevent shingles. So what does it mean when you take away natural infections with mass vaccination? You get more shingles cases.
Interestingly, the January 29, 1999, edition of the CDC’s MMWR indicated that surveillance requirements will become necessary as the vaccine is introduced to help identify needs for “intensive outbreak control.” 45


The previously cited “Successes and challenges” review observed that the effect of “endogenous boosting,” natural exposure to chickenpox helping the immune system, “might have been underestimated” by medical and pharmaceutical scientists. The authors say supporting evidence suggests that asymptomatic reactivation of the virus happens more frequently than realized, which boosts the individual’s immune system and benefits those around him with a similar exposure boost.
Interestingly, it has been observed “that pediatricians have enhanced protection against shingles when compared to the general population, most likely due to periodic reexposure to children with chickenpox.”46
Antelope Valley
So were the scientists with concerns about increased cases of shingles right?
Merck agreed to post-licensure studies to monitor the effect of the vaccine. Gary Goldman, Ph.D., is a computer scientist who served as research analyst on the CDC-funded Antelope Valley Varicella Active Surveillance Project (VASP) for eight years beginning in 1995.
In 1995, Goldman was hired as the lone research analyst on a project called the “Varicella Active Surveillance Project” (VASP). VASP was funded by the CDC and conducted for the Los Angeles County Department of Health. The point was “to monitor the effects of the universal varicella vaccination program on the 300,000 residents comprising the study population.” 47Goldman designed the database and statistical analysis programs for the project. After eight years on the job, he quit “to avoid participating in what he perceived was research fraud.”
Remember that the “varicella-zoster virus” is linked to both chickenpox and shingles.
In 2022, an interview with Goldman was published where he claims, “The CDC obscured the immunologically mediated link between varicella vaccination and HZ [shingles] epidemiology, especially concerning increased HZ incidence rates among individuals with a history of natural varicella. The CDC perpetuated a false narrative regarding the role that universal varicella vaccination played in reducing exposures to wild-type varicella, which provide natural immune boosts helping to prevent or postpone the reactivation of the varicella-zoster virus as HZ.” 48
Goldman reports he was “encouraged to pursue any analyses and studies that might be suitable for publication, but was barred from publishing significant findings that showed negative health outcomes associated with the program.” He had evidence of “malfeasance, research bias or scientific misconduct.”
Goldman explained that his recommendation to collect data on both chickenpox and shingles from the beginning of the study was refused. This means there was no baseline for comparing rates of shingles before and after deployment of chickenpox vaccines. He said this deliberate ignorance of the baseline information was “either incompetence or misconduct by health authorities, especially since the FDA was fully aware the HZ [shingles] rates were likely to increase.”
Goldman stated that the SBA document from 1995 acknowledged the possibility of increased incidents of shingles due to the widespread use of the varicella vaccine. But its licensure was justified in the savings of time lost at work.49 Despite the known concern, the initial Phase IV study he launched did not include surveillance for shingles, only chickenpox.
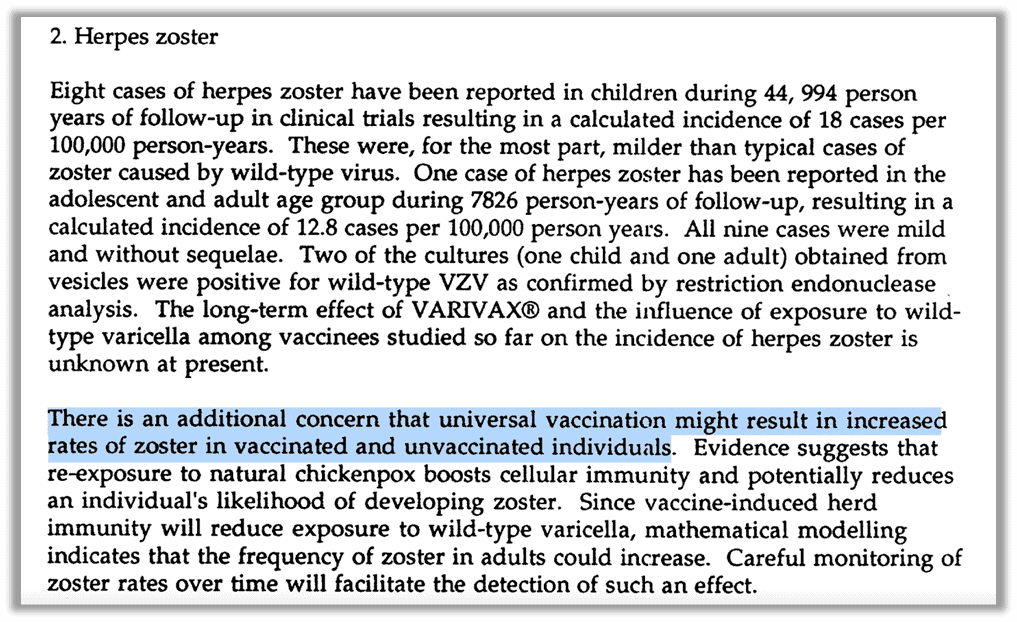
Goldman stated that “although CDC approved HZ active surveillance starting January 1, 2000, a lack of HZ surveillance from the inception of this project is suggestive of either incompetence or misconduct by health authorities, especially since the FDA was fully aware that HZ rates were likely to increase following universal varicella vaccination.” He references a report written by FDA scientists and published in “The Journal Pediatrics” also indicating the known concern:
“The incidence of zoster in vaccinated and unvaccinated individuals might increase after universal immunization. There is evidence that reexposure to natural chickenpox boosts cellular immunity and potentially reduces an individual’s likelihood of having zoster. Vaccine-induced herd immunity will reduce exposure to wild-type varicella; Mathematical modeling indicates that the frequency of zoster in adults could increase.” 50
Goldman asserts that the data he collected indicated increased incidents of shingles in young children as young as 10. The publications being made by the CDC on data he worked on lacked integrity and were misleading. We strongly encourage anyone interested in this to go to the interview directly for a deeper dive on a first-person account of manipulation of published science and messaging from our own federal government. Goldman’s research was suppressed and he was eventually told to stop studying shingles. After resigning, Goldman went through legal battles to be able to use the data from the publicly funded study in publications he felt strongly needed to be informed.


What’s in the vaccine?
The SBA document Merck submitted to the FDA for licensure tells us the vaccine contains the attenuated virus, plus “sucrose, hydrolyzed gelatin, sodium chloride, monosodium L-glutamate [MSG], sodium phosphate dibasic, potassium phosphate monobasic, potassium chloride, residual components of MRC-5 cells including DNA and protein, and trace quantities of sodium phosphate monobasic, EDTA, neomycin, and fetal bovine serum.”51 (emphasis added)
The SBA describes a “robotic” process of sterilization throughout manufacturing, which includes batch testing for “absence of viral adventitious agents,” and reports no rogue viral agents. The “adventitious agents” are unintentional contaminants, and it is known that for live-virus vaccines, it may not be possible to remove them all. 52
One world-famous scientist in a position to understand the devastating possibilities of the inability to detect and filter all contaminants is Albert Sabin, creator of one of the earliest polio vaccines. Polio vaccines were notoriously contaminated, as stories of the Cutter Incident and the SV-40 debacle describe. JAMA quoted Sabin to describe the safety concerns with the varicella vaccine: “for every agent you know about and screen for, there are always others that you don’t know about and so cannot screen for.”

JAMA published a perspective on the “Lengthy Tale of Varicella Vaccine Development…” when the vaccine was licensed, specifically pointing out the concerns with the DNA contamination.53 Varicella is difficult to work with because it stays buried in cells when it is “grown” in labs, requiring scientists to bust up the cell with sound waves to get it out. This smash-and-grab leads to more contamination than is expected in something like the rubella vaccine, which also uses MRC-5 fetal cells. JAMA reports that the FDA advisory committee vetting the vaccine met in closed session to discuss “the possibility of autoimmune reaction” due to the DNA contamination. But the vaccine was still given a green light, with FDA’s Krause telling VRBPAC, “While this study would not detect very rare events in vaccines, it provides some level of assurance.” (emphasis added) And that was good enough for government work, as they say.
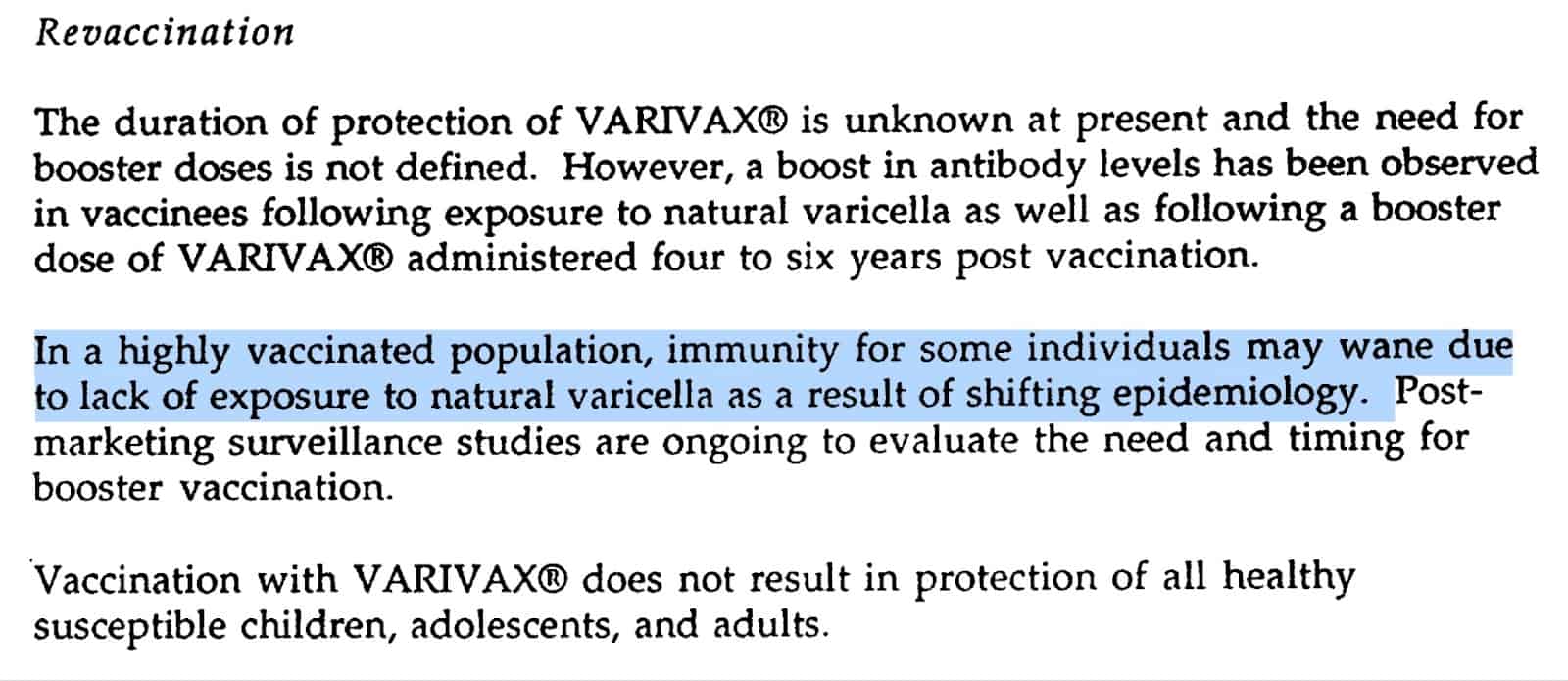
In 1995, the FDA approved VARIVAX, the first varicella vaccine by Merck. The SBA54 document published by Merck reveals a plethora of troubling information, including the acknowledgment of the significant shift in epidemiology that would occur with widespread use of the vaccine, as the reduced exposure to natural varicella could lead to waning immunity in some individuals. Should the product prove effective, it would potentially leave hundreds of millions of people vulnerable to a more serious illness: shingles.
Source: Merck’s 1995 SBA for VARIVAX
Incredibly, the Merck document reveals that while the duration of protection provided by VARIVAX is unknown, a boost in immunity is provided by natural exposure to varicella as well as following a booster dose of the vaccine “administered four to six years post vaccination.”55
Contamination with fetal cell DNA higher than other vaccines
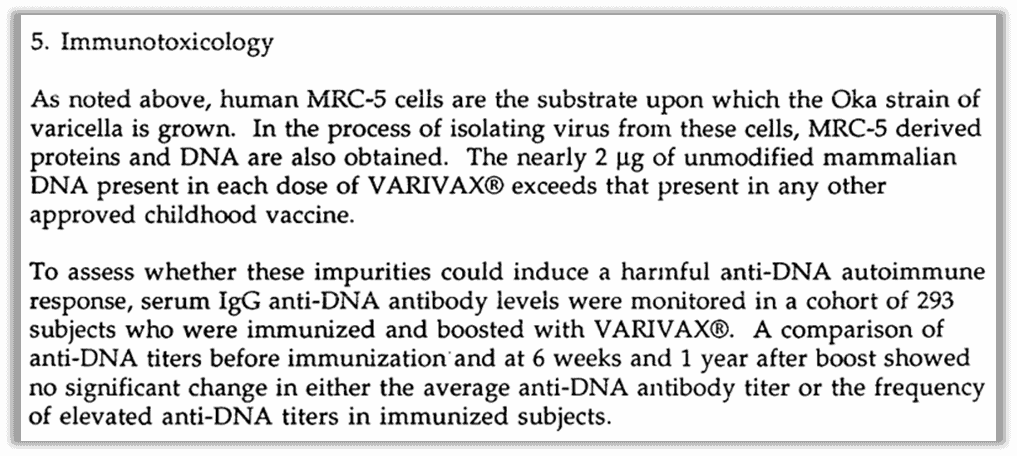
What do patients know about this before getting a chickenpox vaccine? The package insert for the shot is lacking the section typically covering immunotoxicity. The missing Section 13 would address it. At least one website that promotes vaccines, Vaxopedia, claims that other shots might not have this section because the FDA doesn’t require it for older vaccines. But upon reviewing the FDA’s website, we found that almost every other vaccine has this section in its product insert. The exceptions are one of the available HIB vaccines, and the 23-valent pneumococcal vaccine. Both are newer vaccines, so why they don’t have the section is unclear. We don’t have an answer for this; all we can do is report what’s there.

Conclusion
Let’s talk about the greater good and herd immunity. Is it actually helping our communities to vaccinate our children against chickenpox? When we have strong, persistent, and repeated evidence that the more we use the vaccine, the later in life people will get sick and the worse the outcome will be, does vaccinating really make sense?
When a parent goes to a pediatrician for a shot for their child, is this discussed in the risk-benefit conversation? (And is there even a risk-benefit conversation?) Is this information printed on the vaccine information statement? No. Is the parent informed that there’s a mutated fetal cell line and DNA contamination in the shot in a higher concentration than in other vaccines? No.
How can a parent give true informed consent to a chickenpox vaccine when this kind of information is absent? Do parents know they’re consenting to a higher risk of hospitalization, death, or pregnancy complications if their child gets chickenpox later in life? Do they know they’re consenting to seeing more of our older adults suffer from shingles? Do their pediatricians even know this?
Before the vaccine, the number of people who got chickenpox each year equaled the number of people born. Everyone got chickenpox. This should give us pause. No matter what you believe about our creation as humans, this is significant. Our bodies were designed to have chickenpox as young children. Chickenpox didn’t decimate the human population. There aren’t any stories of chickenpox plagues. Instead, we contract it, we heal, and we thrive. As children – as our immune systems are developing – humans universally endure itchy bumps and a mild fever that passes quickly and gives us lifelong immunity.
Up until the time the vaccine was released on the world, chickenpox was a childhood rite of passage, so well-known and mild that there are “silly” children’s stories about it. If you, yourself, did not have chickenpox, you know someone who did – maybe a parent or other family member. Why? Why would every human go through this? Is it a random germ war, where a villainous virus attacks vulnerable innocents for no other reason than its own survival… or is there something else afoot?
So we’ll leave you with the same question we asked when we began: “If you could prevent shingles, why wouldn’t you?”
References
- “Chapter 22: Varicella.” CDC Pink Book, (2021). https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-22-varicella.html. ↩︎
- “Varicella-Related Deaths Among Adults — United States, 1997.” CDC MMWR 46, no. 19 (1977): 409-412. https://www.cdc.gov/mmwr/preview/mmwrhtml/00047618.htm. ↩︎
- “Management of Varicella Infection (Chickenpox) in Pregnancy.” Journal of Obstetrics and Gynaecology Canada, no. 274 (2012): 287-292. https://www.jogc.com/article/S1701-2163(16)35190-8/pdf#:~:text=10%20In%20one%20prospective%20study,required%20intubation%20and%20mechanical%20ventilation. ; “Successes and Challenges in Varicella Vaccine.” Therapeutic Advances in Vaccines and Immunotherapy 2, no. 2 39–55. https://pmc.ncbi.nlm.nih.gov/articles/PMC3991154/. ↩︎
- Fishbein, Morris M.D. 1949. Modern Home Medical Adviser. Doubleday. https://www.amazon.com/Modern-Medical-Adviser-Morris-Fishbein/dp/B000RQTIVO. ↩︎
- Czinn, Amber B. BS, MS2, and Leonard J. Hoenig MD. “Poxes Great and Small: The Stories behind Their Names.” Clinics in Dermatology 41, no. 3 (2023): 459-462. https://pmc.ncbi.nlm.nih.gov/articles/PMC9997050/. ↩︎
- Czinn, Amber B. BS, MS2, and Leonard J. Hoenig MD. “Poxes Great and Small: The Stories behind Their Names.” Clinics in Dermatology 41, no. 3 (2023): 459-462. https://pmc.ncbi.nlm.nih.gov/articles/PMC9997050/. ↩︎
- Galetta, Kristin M., and Don Gilden. “Zeroing in on Zoster: A Tale of Many Disorders Produced by One Virus.” Journal of the Neurological Sciences 358, no. 1-2 (2015): 38–45. https://pmc.ncbi.nlm.nih.gov/articles/PMC4628852/pdf/nihms-729318.pdf. ↩︎
- Czinn, Amber B. BS, MS2, and Leonard J. Hoenig MD. “Poxes Great and Small: The Stories behind Their Names.” Clinics in Dermatology 41, no. 3 (2023): 459-462. https://pmc.ncbi.nlm.nih.gov/articles/PMC9997050/. ↩︎
- Galetta, Kristin M., and Don Gilden. “Zeroing in on Zoster: A Tale of Many Disorders Produced by One Virus.” Journal of the Neurological Sciences 358, no. 1-2 (2015): 38–45. https://pmc.ncbi.nlm.nih.gov/articles/PMC4628852/pdf/nihms-729318.pdf. ↩︎
- “Chapter 22: Varicella.” CDC Pink Book, (2021). https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-22-varicella.html. ↩︎
- “Chapter 22: Varicella.” CDC Pink Book, (2021). https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-22-varicella.html. ↩︎
- “Itchy, Itchy, Chicken Pox!” Ms Kelly Online. Video, https://www.youtube.com/watch?v=-nmbkMAWDig.
↩︎ - “Evaluation of Varicella Reporting to the National Notifiable Disease Surveillance System — United States, 1972-1997.” CDC MMWR 43, no. 3 (1999): 55-58. https://www.cdc.gov/mmwr/preview/mmwrhtml/00056339.html. ↩︎
- “Chapter 22: Varicella.” CDC Pink Book, (2024). https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-22-varicella.html. ↩︎
- Galetta, Kristin M., and Don Gilden. “Zeroing in on Zoster: A Tale of Many Disorders Produced by One Virus.” Journal of the Neurological Sciences 358, no. 1-2 (2015): 38–45. https://pmc.ncbi.nlm.nih.gov/articles/PMC4628852/pdf/nihms-729318.pdf. ↩︎
- Galetta, Kristin M., and Don Gilden. “Zeroing in on Zoster: A Tale of Many Disorders Produced by One Virus.” Journal of the Neurological Sciences 358, no. 1-2 (2015): 38–45. https://pmc.ncbi.nlm.nih.gov/articles/PMC4628852/pdf/nihms-729318.pdf. ↩︎
- Galetta, Kristin M., and Don Gilden. “Zeroing in on Zoster: A Tale of Many Disorders Produced by One Virus.” Journal of the Neurological Sciences 358, no. 1-2 (2015): 38–45. https://pmc.ncbi.nlm.nih.gov/articles/PMC4628852/pdf/nihms-729318.pdf. ↩︎
- “Chapter 23: Zoster.” CDC Pink Book, (2024). https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-23-zoster.html. ↩︎
- “Shingles.” Cleveland Clinic, (2024). https://my.clevelandclinic.org/health/diseases/11036-shingles. ↩︎
- “Shingles.” Cleveland Clinic, (2024). https://my.clevelandclinic.org/health/diseases/11036-shingles. ↩︎
- “Chapter 22: Varicella.” CDC Pink Book, (2024). https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-22-varicella.html. ↩︎
- “VARIVAX: Reconstitution.” MerckVaccines.Com,. https://www.merckvaccines.com/varivax/diluent-reconstitution/. ↩︎
- “VARIVAX Package Insert.” (2023). https://www.fda.gov/media/76000/download. ↩︎
- Allen, Arthur. 2008. Vaccine: The Controversial Story of Medicine’s Greatest Lifesaver. W. W. Norton & Company, page 218. ↩︎
- Allen, Arthur. 2008. Vaccine: The Controversial Story of Medicine’s Greatest Lifesaver. W. W. Norton & Company ↩︎
- Preblud, S R. “Varicella: Complications and Costs.” Pediatrics 78, no. 4 pt. 2 (1986): 728-735. https://pubmed.ncbi.nlm.nih.gov/3093966/. ↩︎
- Allen, Arthur. 2008. Vaccine: The Controversial Story of Medicine’s Greatest Lifesaver. W. W. Norton & Co. https://www.amazon.com/Vaccine-Controversial-Medicines-Greatest-Lifesaver/dp/0393331563. ↩︎
- “Successes and Challenges in Varicella Vaccine.” Therapeutic Advances in Vaccines and Immunotherapy, (2014). https://journals.sagepub.com/doi/10.1177/2051013613515621. ↩︎
- Marwick, Charles. “Lengthy Tale of Varicella Vaccine Development Finally Nears a Clinically Useful Conclusion.” JAMA 273, no. 11 (1995): 833-836. https://jamanetwork.com/journals/jama/article-abstract/387439. ↩︎
- White, C. Jo . “Varicella-Zoster Virus Vaccine.” Clinical Infectious Diseases 24, no. 5 (1997): 753-761. ↩︎
- Yardley, William . “Michiaki Takahashi, 85, Who Tamed Chickenpox, Dies.” The New York Times, December 21, 2013. https://www.nytimes.com/2013/12/22/health/michiaki-takahashi-85-who-tamed-chickenpox-dies.html. ↩︎
- Gershon, Anne A. “Live Attenuated Varicella-Zoster Vaccine.” Reviews of Infectious Diseases 2, no. 3 (1980): 393-407. https://www.jstor.org/stable/4452445. ↩︎
- White, C. Jo . “Varicella-Zoster Virus Vaccine.” Clinical Infectious Diseases 24, no. 5 (1997). https://academic.oup.com/cid/article-abstract/24/5/753/478600. ↩︎
- Kamiya, Hitoshi, et.al. “Diagnostic Skin Test Reactions with Varicella Virus Antigen and Clinical Application of the Test.” The Journal of Infectious Diseases 136, no. 6 (1977): 784-788. https://sci-hub.st/10.2307/30107059. ↩︎
- Gershon, Anne A. “Perspective: Live Attenuated Varicella Vaccine.” The Journal of Infectious Diseases 152, no. 5 (1985): 859-862. https://www.jstor.org/stable/30104716. ↩︎
- Marwick, Charles. “Lengthy Tale of Varicella Vaccine Development Finally Nears a Clinically Useful Conclusion.” JAMA 273, no. 11 (1995): 833-836. ↩︎
- Marwick, Charles. “Lengthy Tale of Varicella Vaccine Development Finally Nears a Clinically Useful Conclusion.” JAMA 273, no. 11 (1995): 833-836. ↩︎
- Gershon, Anne A., and Michael D. Gershon. “Perspectives on Vaccines Against Varicella-Zoster Virus Infections.” Current Topics in Microbiology and Immunology 342, (2017): 359–372. https://pmc.ncbi.nlm.nih.gov/articles/PMC5391036/. ↩︎
- “About the Varicella Vaccines.” CDC Vaccines and Immunizations, (2021). https://www.cdc.gov/vaccines/vpd/varicella/hcp/about-vaccine.html. ↩︎
- “Successes and Challenges in Varicella Vaccine.” Therapeutic Advances in Vaccines and Immunotherapy 2, no. 2 39–55. https://pmc.ncbi.nlm.nih.gov/articles/PMC3991154/. ↩︎
- Wang, Wei, et.al. “Development of a Skin- and Neuro-attenuated Live Vaccine for Varicella.” Nature Communications 13, (2022). https://www.nature.com/articles/s41467-022-28329-1. ↩︎
- “Varicella (Chickenpox).” Government of Canada, (2023). https://www.canada.ca/en/public-health/services/immunization/vaccine-preventable-diseases/varicella-chickenpox.html. ;
“Successes and Challenges in Varicella Vaccine.” Therapeutic Advances in Vaccines and Immunotherapy 2, no. 2 39–55. https://pmc.ncbi.nlm.nih.gov/articles/PMC3991154/. ↩︎ - Cowan , Thomas MD. 2018. Vaccines, Autoimmunity, and the Changing Nature of Childhood Illness. Chelsea Green Publishing. https://www.amazon.com/Vaccines-Autoimmunity-Changing-Childhood-Illness/dp/1603587772. ↩︎
- Vaccine 20, no. 19 (2002). https://www.sciencedirect.com/journal/vaccine/vol/20/issue/19. (graphic below paragraph) ↩︎
- “Evaluation of Varicella Reporting to the National Notifiable Disease Surveillance System — United States, 1972-1997.” CDC MMWR 43, no. 3 (1999): 55-58. https://www.cdc.gov/mmwr/preview/mmwrhtml/00056339.htm. ↩︎
- Miller, Neil Z., and Gary S. Goldman. “Interview with Gary Goldman, PhD on CDC Suppression of Undesirable Vaccine Data.” Foundation for Educational Research, (2022). http://thinktwice.com/interview-with-Gary-Goldman.pdf. ↩︎
- Miller, Neil Z., and Gary S. Goldman. “Interview with Gary Goldman, PhD on CDC Suppression of Undesirable Vaccine Data.” Foundation for Educational Research, (2022). http://thinktwice.com/interview-with-Gary-Goldman.pdf. ↩︎
- Miller, Neil Z., and Gary S. Goldman. “Interview with Gary Goldman, PhD on CDC Suppression of Undesirable Vaccine Data.” Foundation for Educational Research, (2022). http://thinktwice.com/interview-with-Gary-Goldman.pdf. ↩︎
- Lieu, T A, et.al. “Cost-effectiveness of a Routine Varicella Vaccination Program for US Children.” JAMA 271, no. 5 (1994): 375-381. https://pubmed.ncbi.nlm.nih.gov/8283587/. ↩︎
- Krause, P R., and D M. Klinman. “Efficacy, Immunogenicity, Safety, and Use of Live Attenuated Chickenpox Vaccine.” The Journal of Pediatrics 127, no. 4 (1995): 518-525. https://pubmed.ncbi.nlm.nih.gov/7562270/. ↩︎
- “Summary For Basis Of Approval: VARIVAX.” Merck and Co., (1995). https://s3.amazonaws.com/soundchoice/soundchoice/wp-content/uploads/2013/08/Appendix-F-Varivax-Summary-for-Basis-of-Approval.pdf. ↩︎
- Klug, Bettina, et.al. “Adventitious Agents and Live Viral Vectored Vaccines: Considerations for Archiving Samples of Biological Materials for Retrospective Analysis.” Vaccine 34, no. 51 (2016): 6617–6625. https://pmc.ncbi.nlm.nih.gov/articles/PMC5130882/.
↩︎ - Marwick, Charles. “Lengthy Tale of Varicella Vaccine Development Finally Nears a Clinically Useful Conclusion.” JAMA 273, no. 11 (1995): 833-836. ↩︎
- “Summary For Basis Of Approval: VARIVAX.” Merck and Co., (1995). https://s3.amazonaws.com/soundchoice/soundchoice/wp-content/uploads/2013/08/Appendix-F-Varivax-Summary-for-Basis-of-Approval.pdf. ↩︎
- “Summary For Basis Of Approval: VARIVAX.” Merck and Co., (1995). https://s3.amazonaws.com/soundchoice/soundchoice/wp-content/uploads/2013/08/Appendix-F-Varivax-Summary-for-Basis-of-Approval.pdf. ↩︎



